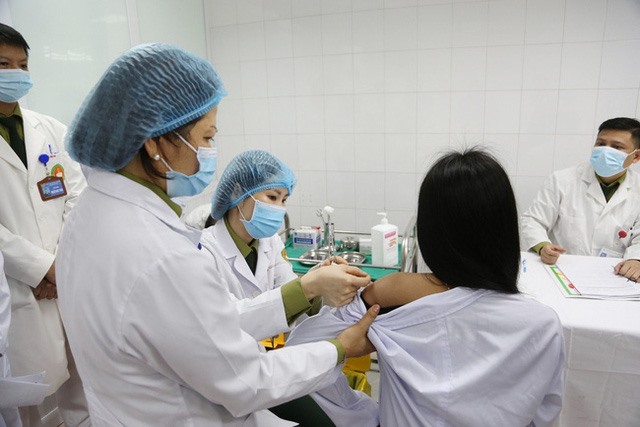
HCMC – Ho Nhan, general director of Nanogen Pharmaceutical Biotechnology JSC, said there were no serious side effects or fatalities reported among 14,000 volunteers during the human trials of the Nano Covax Covid-19 vaccine.
Speaking at a meeting with State President Nguyen Xuan Phuc in HCMC on July 29, Nhan said the locally developed Covid-19 vaccine has undergone seven months of clinical trials through three phases —1, 2 and 3A.
The vaccine has proved to be safe and effective against the coronavirus, including the Delta variant.
Nhan said some 30 countries, including the world’s leading vaccine producer, India, have asked for the technology transfer from Nanogen. The World Health Organization is also evaluating the company’s vaccine dossier.
The company is currently capable of producing over 10 million doses of the Nano Covax Covid-19 vaccine a month and would raise its capacity to 30 million doses a month in October.
Nhan suggested the Government grant conditional approval for the emergency use of Nano Covax. Nanogen will soon begin phases 3B and 4 of the clinical trials for the vaccine.
State President Nguyen Xuan Phuc spoke highly of Nanogen’s efforts in developing a domestic Covid-19 vaccine in the context of the complicated developments of the pandemic and the global vaccine scarcity.
“We must consider vaccine production as an urgent task of national importance. Vaccination and social distancing are the country’s top solutions for Covid-19 infection prevention and control,” he said.
The State President asked the Ministry of Health and competent agencies to create favorable conditions for the development and production of domestic Covid-19 vaccines.
They should work with vaccine manufacturers to accelerate the development of Covid-19 vaccines but safety must be the top priority.
Besides, Phuc and Prime Minister Pham Minh Chinh have approved a proposal to invite experts from WHO to evaluate the safety and efficacy of locally developed Covid-19 vaccines.